- Randomized clinical study or a prospective study with a well-defined control group.
- Defined diagnosis and endpoints.
- Diagnostic reliability tests and reproducibility tests described.
- Blinded outcome assessment.
- Cohort study or retrospective cases series with defined control or reference group.
- Defined diagnosis and endpoints.
- Diagnostic reliability tests and reproducibility tests described.
- Large attrition.
- Unclear diagnosis and endpoints.
- Poorly defined patient material.
The Grading of Recommendations Assessment, Development and Evaluation (GRADE) [16] was implemented to assess the overall quality of evidence for the studies included in this systematic review, according to which the overall evidence is rated as high, moderate, low, and very low. The outcomes included in GRADE were divided into categories regarding the different parameters that had been assessed in the primary studies.
- High quality of evidence implies that the true effect lies close to that of the estimate of the effect
- Moderate quality of evidence implies that the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different
- Low quality of evidence implies that our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect
- Very low quality of evidence implies that the true effect is likely to be substantially different from the estimate of effect.
Results
Study selection
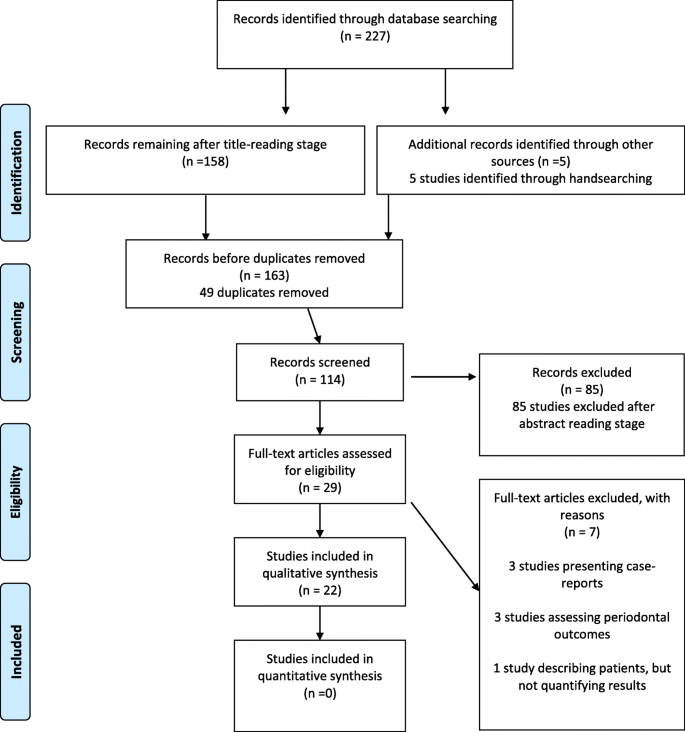
The electronic search initially identified 227 relevant articles. One hundred fifty-eight papers remained after exclusion on the basis of title-reading. Five articles were added through hand-searching. After 49 duplicates’ removal, 114 papers were assessed for screening, and after abstract-reading, 85 studies were excluded leaving 29 articles to be read in full-text. After the application of specific inclusion and exclusion criteria, another seven articles were removed. In total, 22 studies were considered eligible for inclusion in the final analysis (Fig. 1).
Study characteristics
The characteristics of each study are presented in detail in Table 1. Table 2 gives an overview of the results of the included studies regarding clinical parameters. Three studies [18,19,20] were RCTs, eight studies were of prospective [5, 21,22,23,24,25,26,27], and 11 of retrospective design [28,29,30,31,32,33,34,35,36,37,38].
Table 1 An overview of the included studies providing information on the experimental designs and settings
Table 2 Overview of the results, outcomes, and conclusions of the included studiesQuality analysis
The quality assessment of the 22 studies is shown in Tables 3 and 4.
Table 3 Quality assessment of the included RCT studies Table 4 Quality assessment of the included prospective and retrospective studiesRCTs
The three RCTs [18,19,20] were judged to be at an overall low risk of bias, due to the low risk of bias that applied to each domain based on the Cochrane risk of bias tool [16] (Table 3).
Prospective studies
Three prospective studies [21, 26, 35] were graded as moderate and five [5, 22, 24, 25, 27] as high risk of bias. Although they were all studies of prospective design, no blinding in relation to outcome assessment was reported in all except one [27] study, which also lacked control, among other limitations (Table 4).
Retrospective studies
Ten out of the 11 identified retrospective studies [28,29,30,31,32,33,34,35,36,37,38] were graded as moderate risk of bias, since all the pre-determined criteria were met. Only one retrospective study [34] was of high risk of bias, because it did not include any diagnostic reliability and reproducibility tests (Table 4).
Qualitative synthesis of the included studies
Study settings
An overview of the experimental design of the included studies is presented in Table 1. Eight studies [5, 21, 22, 24, 30, 34,35,36] used patients’ virtual ClinCheck® models of the predicted tooth movement as control group, aided by ToothMeasure® [5, 21, 22, 24, 34,35,36] or Geomagic Qualify [30], in order to investigate the treatment’s efficacy. More specifically, the extent that the initial and final actual models were different from the initial and final virtual models after treatment was evaluated. However, two of them had similar samples and outcomes with two other studies, namely [5] with [24, 35] with [36]. We decided not to exclude any of these studies, since additional information was provided. Seven studies [18, 19, 23, 28, 33, 37, 38] compared treatment outcome of Invisalign® orthodontic treatment with that of conventional fixed appliances. At last, four studies [20, 25, 29, 32] compared Invisalign® groups to each other, while one study [31] did not have any control or comparison group.
All studies tested mainly non-growing patients, and most of them included patients of an average age of 30 years [5, 19,20,21, 29,30,31, 34,35,36,37,38]. Non-extraction cases were used as study samples in nine studies [18, 28,29,30,31,32,33, 37, 38]. Treatment duration differed among and within studies, as expected according to malocclusion severity and the implemented intervention. Six studies [18, 22, 29, 34,35,36] did not report on treatment duration. Finally, only one study [37] reported post-retention treatment outcomes by comparing the induced changes in patients treated with Invisalign® with those treated with traditional fixed appliances. The evaluation was conducted at a maximum post-retention time of 3 years after appliance removal, with all the patients undergoing at least 1 year of retention.
Clinical findings
Table 2 gives an overview of the results of the included studies regarding clinical parameters, grouped in the following three subject categories.
A. Accuracy
The accuracy of Invisalign® was reported in nine studies [5, 21, 22, 24, 26, 30, 34,35,36], where it was evaluated as the deviation between the achieved and the planned tooth movements. The findings among studies were varying ranging from sufficient accuracy in resolving anterior crowding [35, 36] and distalizing maxillary molars [34] to contradictory findings in upper incisor root control [22, 34] and to inadequacies in bodily expansion of the maxillary posterior teeth [21, 26, 30], canine [5, 24] and premolar [34] rotational movements, extrusion of maxillary incisors5, and in overbite control [35, 36].
B. Invisalign® vs traditional fixed appliances
Seven studies [18, 19, 23, 28, 33, 37, 38] compared Invisalign® orthodontic treatment outcomes to that of conventional fixed appliances. A recent RCT study [18] found no significant difference in the amount of mandibular incisor proclination produced by Invisalign® and fixed labial appliances in mild crowding cases, supported by a retrospective study [23], which also concluded that treatment duration in these cases was similar for the two methods, though Invisalign was not so successful in root alignment. Gu et al. [28] reported similar outcomes, but shorter duration with Invisalign, for mild to moderate malocclusions. However, worse performance of Invisalign was noted in more severe cases, a finding also supported by Djeu et al. [38]. In the same line, in a RCT study, Li et al. [19] concluded that both therapeutic approaches can succeed in class I adult extraction cases, though Invisalign required more time and was less able to correct bucco-lingual inclination and occlusal contacts. The latter findings are also in agreement with those of two retrospective studies [33, 38].
Differences between the two methods in post-retention alterations were investigated in one retrospective moderate risk of bias study [37]. Greater relapse was found 1–3 years posttreatment after Invisalign® treatment compared to conventional orthodontic therapy with fixed appliances.
C. Invisalign groups only
In an early exploratory study, Vlaskalic and Boyd [25] concluded that Invisalign® may be more beneficial for patients in the permanent dentition with mild to moderate malocclusions after careful treatment planning. Another early exploratory RCT study [20] also concluded that non-extraction treatment of milder malocclusions has greater chances to be successfully treated by Invisalign.
Three recent retrospective studies also tested various Invisalign groups. One showed the moderate ability of Invisalign to manage overbite [29]. More specifically, normal overbite was well maintained, but deep bite was partially corrected, through mandibular incisor proclination. Open bite was also partially corrected, but mainly through incisor extrusion. On the other hand, a second study [31] reported the ability of Invisalign to bodily distalize maxillary molars in adult nonextraction mild class II cases (≤ ½ cusp), with no changes in facial height. Finally, a third study [32] showed the ability of Invisalign to correct mild to moderate crowding nonextraction cases without causing significant changes in the mandibular incisor position and inclination. On the contrary, such changes (protrusion and proclination) were induced in cases with severe crowding (≥ 6 mm).
The Grading of Recommendations Assessment, Development and Evaluation (GRADE) [16] was implemented to assess the overall quality of evidence for the studies included in this review and for outcomes that were assessed by two or more studies. GRADE tables illustrate the outcomes that were assessed by two or more studies (Additional file 1, 2, and 3).
Quantitative synthesis of the included studies
The lack of standardized protocols impeded a valid interpretation of the actual results through pooled estimates. Substantial differences in the implemented interventions, participants’ characteristics (age and gender distribution), treatment duration, and investigated outcomes indicated significant methodological heterogeneity. Therefore, a meta-analysis was not feasible.
Discussion
In order to successfully deliver orthodontic treatment, clinicians need to carefully plan an appropriate therapeutic approach based on the current scientific evidence. Although this is not the only determining factor for the final decision, as clinical experience and patient’s opinion also play an important role, this information needs to be taken into consideration to assess the possibilities and limitations of each treatment modality.
With regard to Invisalign®, to date, there are four systematic reviews available, pertaining to clinical effects of the system [12,13,14,15], with one of them [14] evaluating periodontal health issues. Given the limited available evidence in certain earlier attempts [12, 15] and the evaluation of the effectiveness of Invisalign® under the wider spectrum of clear aligners [13, 15], strong conclusions regarding the investigated clinical efficiency of the Invisalign® system were not feasible. This ambient obscurity on a highly increasing treatment approach was the reason to perform a systematic search of the literature and assess the available scientific evidence with respect to the clinical outcomes of the Invisalign® orthodontic treatment. Due to the relatively unexplored topic, an attempt was made to conduct the present systematic review to a high standard, in order to minimize any chance of bias, but also include all the available information.
Indeed, a considerable number of studies were included in this review, though only three of them were RCTs [18,19,20], with low risk of bias. From the remaining 19 studies, 8 were of prospective [5, 21,22,23,24,25,26,27] and 11 of retrospective design [28,29,30,31,32,33,34,35,36,37,38] with moderate [21, 23, 26, 28,29,30,31,32,33,34,35,36,37,38] or high [5, 22, 24, 25, 27, 34] risk of bias. Thus, since it was difficult to assess the outcomes and reach safe results and conclusions, a strict methodology in both the data extraction and quality analysis was attempted. The methodological quality of the retrieved studies was thoroughly evaluated and a qualitative synthesis of the results was performed.
Considerable differences in participants’ characteristics, types of interventions, reporting of clinical outcomes, and treatment’s duration was evident, thus, preventing the implementation of a meta-analysis. More specifically, the number of patients recruited ranged from 6 [22] to 152 [19], which indicates a strong methodological difference among the study protocols and in strength of the stated results. Concerning the age of the patients that underwent treatment with Invisalign®, it varied between 13 [34] and 61 [30] years, with all studies primarily including non-growing patients, most of them having an average age of 30 years [5, 19,20,21, 29,30,31, 34,35,36,37,38], and most of them with moderate [21, 29,30,31, 35,36,37,38] and high [5, 34] risk of bias. This reveals a strong lack of information for growing individuals and indicates that Invisalign® is at present a preferred treatment option for late adolescent and adult patients, who usually have higher esthetic demands.
With regard to the outcome measures, measurements in pre- and post-treatment records were made. The records included the following: actual or/and digital dental casts [5, 19,20,21,22,23, 25, 28, 30, 32, 35,36,37,38], panoramic radiographs [25, 37, 38], lateral cephalograms [18, 19, 25, 29, 31, 32, 38], CBCTs [33], and photographs [19, 20, 25, 38]. The discrepancy index (DI) and the peer assessment rating index (PAR) were used in the pre-treatment records to assess the initial severity of malocclusion [5, 19, 28, 38]. The American Board of Orthodontics – Objective-grading system (ABO-OGS) was used in three studies [5, 19, 38] to systematically grade both pre- and post-treatment records evaluating various clinical parameters. ToothMeasure®, which is the Invisalign®’s proprietary superimposition software, was also used to make measurements on 3D dental models, including the initial and final ClinCheck® virtual models [5, 24, 35, 36].
As for the overall treatment duration, there were different completion criteria and varying outcomes among and within studies. When compared to conventional appliances, the Invisalign® system showed significantly shorter treatment duration in three studies [28, 33, 38], while no difference was reported in another study [23]. All these studies evaluated nonextraction treatment of mild to moderate malocclusions and scored as moderate risk of bias. On the contrary, one study on extraction treatment reported longer duration for Invisalign treatment [19], with low risk of bias. Thus, it seems that Invisalign might treat faster mild nonextraction cases, but it requires more time than fixed appliance treatment for more complex cases.
Substantial variation in the investigated clinical outcomes was noted among studies. The majority of them focused on the accuracy of Invisalign® or its comparison to conventional fixed appliances. The first was found sufficient when certain malocclusion features, such as overjet or anterior arch length discrepancy, were tested [35, 36] or for maxillary molar distalization [34]. The efficacy on maxillary molar distalization (≤ ½ cusp) was also supported by another clinical study [31]. However, important limitations were reported for bodily expansion of the maxillary posterior teeth [21, 30], canine [5, 24] and premolar [34] rotational movements, extrusion of maxillary incisors 5, and in overbite control [35, 36]. All of these referred studies scored as moderate according to Bondemark scoring system [17]. Based on these findings, the use of additional attachments or overcorrections was commonly suggested in the literature for these types of movement. As for the comparison to fixed appliances, from studies with moderate [23, 28] to low [18] risk of bias, it seems that Invisalign performs well in mild to moderate non-extraction cases [18, 23, 28], but it cannot equally succeed in more difficult cases, including extraction cases [19, 27, 28, 33, 38]. Teeth inclinations and occlusal contacts seem to be among the major limitations of Invisalign [19, 33, 38], most of them judged as moderate [23, 33, 38] risk of bias and only two with low [18, 19]. The results from studies that included only different Invisalign groups are in agreement with the abovementioned findings [20, 25, 29, 32].
In addition, only one study [37], graded as moderate, included a post-treatment observational period investigating the stability of treatment outcomes with Invisalign®, indicating a general lack of information with regard to retention. Although the amount of evidence is limited, this study showed more relapse in the Invisalign cases, as compared to fixed appliance treatment, that might be attributed to the inadequacies in obtaining certain bodily movements and solid occlusal contacts.
Overall, evidence was of moderate quality. Apart from the three RCTs [18,19,20], where a low risk of bias was considered, the remaining prospective and retrospective studies were graded as moderate [21, 23, 26, 28,29,30,31,32,33,34,35,36,37,38] or high [5, 22, 24, 25, 27, 34] risk of bias. The studies’ review showed high amount of heterogeneity in terms of methodology and outcome reporting that impeded a valid interpretation of the actual results through pooled estimates. However, there was substantial consistency among researchers that the Invisalign® system is a viable alternative to conventional orthodontic therapy in correcting mild to moderate malocclusions, without extractions. Moreover, when the treatment is carefully planned, Invisalign® aligners can safely straighten dental arches in terms of leveling and derotating the teeth, except for canines and premolars. Finally, crown tipping can be easily performed. On the other hand, important limitations include arch expansion through bodily tooth movements, extraction space closure, corrections of occlusal contacts, and larger antero-posterior and vertical discrepancies.
All things considered, it is evident that more high-quality research of prospective design with respect to the clinical outcomes of Invisalign® needs to be carried out in the future. A standardized methodology including control samples would be valuable in obtaining comparative results with conventional approaches. Furthermore, though more than half of the studies included in the present review have been published in the last 5 years (range 2012–2017), the findings of the review should be interpreted with some caution; the continuous improvement of the Invisalign system (especially in 2013 with SmartTrack® material) [39] may not allow for direct synthesis and valid comparisons between older studies with the most recent ones, as the inclusion of data from different iterations of Invisalign material may become a factor of bias. This is, of course, a major consideration when synthesis of studies’ results for clinical evidence is concerned, in an era that software, scanners, and 3D printer costs are more affordable and potential in-house printing of aligners is rapidly growing. Last but not least, the long-term effectiveness pertaining to retention outcomes also needs further investigation, whereas complete lack of evidence is evident for growing patients.
Conclusions
Despite the fact that orthodontic treatment with Invisalign® is a widely used treatment option, apart from non-extraction treatment of mild to moderate malocclusions of non-growing patients, no clear recommendations about other indications of the system can be made, based on solid scientific evidence.
Although this review included a considerable number of studies, treatment outcomes need to be interpreted with caution due to the high heterogeneity. Further research with parallel arm RCTs or well-designed prospective trials are needed to form robust clinical recommendations for a wide spectrum of malocclusions and for growing patients.
Albeit the existing limitations, the following conclusions were made, based on the available evidence:
- Invisalign might treat faster mild non-extraction cases, but it requires more time than fixed appliance treatment for more complex cases.
- Invisalign® aligners can safely straighten dental arches in terms of leveling and derotating the teeth (except for canines and premolars, where a small inadequacy was reported). Crown tipping can be easily performed.
- Teeth inclinations and occlusal contacts seem to be among the limitations of Invisalign®, when accuracy of planned movements achieved with aligners is concerned.
- Use of additional-novel attachments might be more effective for various types of movement, such as bodily expansion of the maxillary posterior teeth, canine and premolar rotational movements, extrusion of maxillary incisors, and in overbite control.
Abbreviations
American Board of Orthodontics – Objective-grading system
Grading of Recommendations Assessment, Development and Evaluation
Randomized clinical trial
References
- Ziuchkovski JP, Fields HW, Johnston WM, Lindsey DT. Assessment of perceived orthodontic appliance attractiveness. Am J Orthod Dentofacial Orthop. 2008;133(4 Suppl):68–78. ArticleGoogle Scholar
- Rosvall MD, Fields HW, Ziuchkovski J, Rosenstiel SF, Johnston WM. Attractiveness, acceptability, and value of orthodontic appliances. Am J Orthod Dentofac Orthop. 2009;135(3):276e1–12. ArticleGoogle Scholar
- Gkantidis N, Zinelis S, Karamolegkou M, Eliades T, Topouzelis N. Comparative assessment of clinical performance of esthetic bracket materials. Angle Orthod. 2012;82(4):691–7. ArticlePubMedGoogle Scholar
- Boyd RL. Esthetic orthodontic treatment using the Invisalign® appliance for moderate to complex malocclusions. J Dent Educ. 2008;72(8):948–67. PubMedGoogle Scholar
- Kravitz ND, Kusnoto B, BeGole E, Obrez A, Agran B. How well does Invisalign® work? A prospective clinical study evaluating the efficacy of tooth movement with Invisalign®. Am J Orthod Dentofac Orthop. 2009;135(1):27–35. ArticleGoogle Scholar
- Kuo E, Miller RJ. Automated custom-manufacturing technology in orthodontics. Am J Orthod Dentofac Orthop. 2003;123(5):578–81. ArticleGoogle Scholar
- Meier B, Wiemer KB, Miethke RR. Invisalign®-patient profiling. Analysis of a prospective survey. J Orofac Orthop. 2003;64(5):352–8. ArticlePubMedGoogle Scholar
- Aligntech Institute: Tooth movement assessment. Available at: https://s3.amazonaws.com/learn-invisalign/docs/us/ToothAssessment.pdf. Accessed 2016.
- Womack WR. Four-premolar extraction treatment with Invisalign. J Clin Orthod. 2006;40(8):493–500. PubMedGoogle Scholar
- Womack WR, Day RH. Surgical-orthodontic treatment using the Invisalign system. J Clin Orthod. 2008;42(4):237–45. PubMedGoogle Scholar
- Kamatovic M. A retrospective evaluation of the effectiveness of the Invisalign appliance using the PAR and irregularity indices. Toronto: University of Toronto (Canada); 2004. Google Scholar
- Lagravère MO, Flores-Mir C. The treatment effects of Invisalign orthodontic aligners: a systematic review. J Am Dent Assoc. 2005;136(12):1724–9. ArticlePubMedGoogle Scholar
- Rossini G, Parrini S, Castroflorio T, Deregibus A, Debernardi CL. Efficacy of clear aligners in controlling orthodontic tooth movement: a systematic review. Angle Orthod. 2015;85(5):881–9. ArticlePubMedGoogle Scholar
- Rossini G, Parrini S, Castroflorio T, Deregibus A, Debernardi CL. Periodontal health during clear aligners treatment: a systematic review. Eur J Orthod. 2015;37(5):539–43. ArticlePubMedGoogle Scholar
- Zheng M, Liu R, Ni Z, Yu Z. Efficiency, effectiveness and treatment stability of clear aligners: a systematic review and meta-analysis. Orthod Craniofac Res. 2017;20(3):127–33. ArticlePubMedGoogle Scholar
- Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions version 5.1.0, The Cochrane Collaboration, London, UK. http://handbook.cochrane.org (October 2016, date last accessed) 2011.
- Bondemark L, Holm AK, Hansen K, Axelsson S, Mohlin B, Brattstrom V, Paulin G, Pietila T. Long-term stability of orthodontic treatment and patient satisfaction. A systematic review. Angle Orthod. 2007;77(1):181–91. ArticlePubMedGoogle Scholar
- Hennessy J, Garvey T, Al-Awadhi EA. A randomized clinical trial comparing mandibular incisor proclination produced by fixed labial appliances and clear aligners. Angle Orthod. 2016;86(5):706–12. ArticlePubMedGoogle Scholar
- Li W, Wang S, Zhang Y. The effectiveness of the Invisalign appliance in extraction cases using the the ABO model grading system: a multicenter randomized controlled trial. Int J Clin Exp Med. 2015;8(5):8276–82. PubMedPubMed CentralGoogle Scholar
- Bollen AM, Huang G, King G, et al. Activation time and material stiffness of sequential removable orthodontic appliances. Part 1: ability to complete treatment. Am J Orthod Dentofac Orthop. 2003;124(5):496–501. ArticleGoogle Scholar
- Solano-Mendoza B, Sonnemberg B, Solano-Reina E, Iglesias-Linares A. How effective is the Invisalign® system in expansion movement with Ex30’ aligners? Clin Oral Investig. 2017;21(5):1475–84. ArticlePubMedGoogle Scholar
- Castroflorio T, Garino F, Lazzaro A, Debernardi C. Upper-incisor root control with Invisalign appliances. J Clin Orthod. 2013;47(6):346–51. PubMedGoogle Scholar
- Pavoni C, Lione R, Laganà PC. Self-ligating versus Invisalign: analysis of dento-alveolar effects. Ann Stomatol (Roma). 2011;2(1–2):23–7. Google Scholar
- Kravitz ND, Kusnoto B, Agran B, Viana G. Influence of attachments and interproximal reduction on the accuracy of canine rotation with Invisalign®. A prospective clinical study. Angle Orthod. 2008;78(4):682–7. ArticlePubMedGoogle Scholar
- Vlaskalic V, Boyd RL. Clinical evolution of the Invisalign® appliance. J Calif Dent Assoc. 2002;30(10):769–76. PubMedGoogle Scholar
- Buschang PH, Ross M, Shaw SG, Crosby D, Campbell PM. Predicted and actual end-of-treatment occlusion produced with aligner therapy. Angle Orthod. 2015 Sep;85(5):723–7. ArticlePubMedGoogle Scholar
- Baldwin DK, King G, Ramsay DS, Huang G, Bollen AM. Activation time and material stiffness of sequential removable orthodontic appliances. Part 3: premolar extraction patients. Am J Orthod Dentofac Orthop. 2008;133(6):837–45. ArticleGoogle Scholar
- Gu J, Tang JS, Skulski B, Fields HW Jr, Beck FM, Firestone AR, et al. Evaluation of Invisalign treatment effectiveness and efficiency compared with conventional fixed appliances using the Peer Assessment Rating index. Am J Orthod Dentofac Orthop. 2017;151(2):259–66. ArticleGoogle Scholar
- Khosravi R, Cohanim B, Hujoel P, Daher S, Neal M, Liu W, et al. Management of overbite with the Invisalign appliance. Am J Orthod Dentofac Orthop. 2017;151(4):691–9. ArticleGoogle Scholar
- Houle JP, Piedade L, Todescan R Jr, Pinheiro FH. The predictability of transverse changes with Invisalign. Angle Orthod. 2017;87(1):19–24. ArticlePubMedGoogle Scholar
- Ravera S, Castroflorio T, Garino F, Daher S, Cugliari G, Deregibus A. Maxillary molar distalization with aligners in adult patients: a multicenter retrospective study. Prog Orthod. 2016;17:12. ArticlePubMedPubMed CentralGoogle Scholar
- Duncan LO, Piedade L, Lekic M, Cunha RS, Wiltshire WA. Changes in mandibular incisor position and arch form resulting from Invisalign correction of the crowded dentition treated nonextraction. Angle Orthod. 2016;86(4):577–83. ArticlePubMedGoogle Scholar
- Grünheid T, Gaalaas S, Hamdan H, Larson BE. Effect of clear aligner therapy on the buccolingual inclination of mandibular canines and the intercanine distance. Angle Orthod. 2016;86(1):10–6. ArticlePubMedGoogle Scholar
- Simon M, Keilig L, Schwarze J, Jung BA, Bourauel C. Treatment outcome and efficacy of an aligner technique—regarding incisor torque, premolar derotation and molar distalization. BMC Oral Health. 2014;14:68. ArticlePubMedPubMed CentralGoogle Scholar
- Krieger E, Seiferth J, Marinello I, Jung BA, Wriedt S, Jacobs C, et al. Invisalign® treatment in the anterior region were the predicted tooth movements achieved? J Orofac Orthop. 2012;73(5):365–76. ArticlePubMedGoogle Scholar
- Krieger E, Seiferth J, Saric I, Jung BA, Wehrbein H. Accuracy of Invisalign® treatments in the anterior tooth region. First results. J Orofac Orthop. 2011;72(2):141–9. ArticlePubMedGoogle Scholar
- Kuncio D, Maganzini A, Shelton C, Freeman K. Invisalign and traditional orthodontic treatment postretention outcomes using the American Board of Orthodontics objective grading system. Angle Orthod. 2007;77(5):864–9. ArticlePubMedGoogle Scholar
- Djeu G, Shelton C, Maganzini A. Outcome assessment of Invisalign and traditional orthodontic treatment compared with the American Board of Orthodontics objective grading system. Am J Orthod Dentofac Orthop. 2005;128(3):292–8. ArticleGoogle Scholar
- Align Technology. Invisalign for adults and teens. Available at: www.invisalign.com/braces-for-adults-and-teens. Accessed on 25 Aug 2015.
Protocol and registration
The protocol was not registered prior to the study. This study was not registered in any publicly assessable database.
Availability of data and materials
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Author information
Authors and Affiliations
- Department of Orthodontics and Dentofacial Orthopedics, 251 Hellenic Air Force General Hospital, P. Kanellopoulou 3, 11525, Athens, Greece Aikaterini Papadimitriou & Dimitrios Kloukos
- Department of Orthodontics, University Hospital Ghent P8, University of Ghent, C. Heymanslaan 10, B-9000, Ghent, Belgium Sophia Mousoulea
- Department of Orthodontics and Dentofacial Orthopedics, University of Bern, Freiburgstrasse 7, CH-3010, Bern, Switzerland Nikolaos Gkantidis & Dimitrios Kloukos
- Aikaterini Papadimitriou
Contributions
The first two authors (AP and SM) performed data extraction independently and in duplicate. Disagreements were resolved by discussion or the involvement of two collaborators (third author and last author: NG and DK). All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethical approval was not required.
Consent for publication
Competing interests
The authors declare that they have no competing interest.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional files
Additional file 1:
GRADE Working Group grades of evidence. Summary of findings: Invisalign compared in groups of different treatment modalities or divergent severity of crowding. (DOCX 16 kb)
Additional file 2:
GRADE Working Group grades of evidence. Summary of findings: Accuracy of treatment result. (DOCX 16 kb)
Additional file 3:
GRADE Working Group grades of evidence. Summary of findings: Invisalign compared to fixed appliances in adults. (DOCX 16 kb)